 About HPTN 084
About HPTN 084
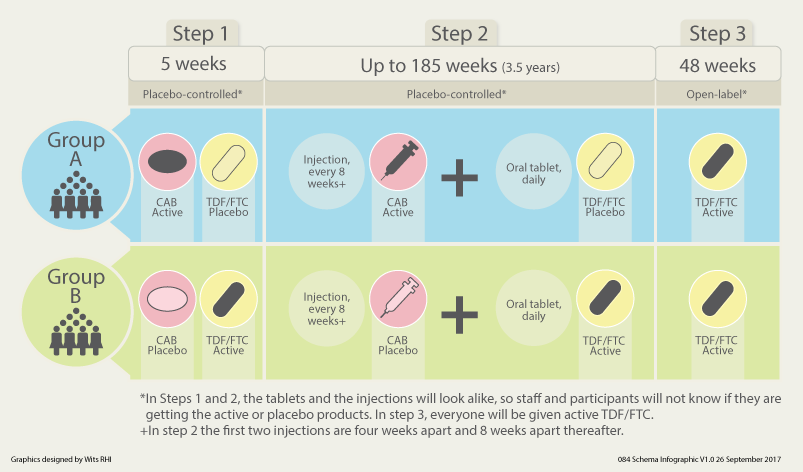
HPTN 084 is the first large-scale clinical trial of a long-acting injectable drug for HIV prevention in women. The study is examining whether a long-acting form of the anti-HIV drug cabotegravir (CAB) injected once every 8 weeks can safely protect women from getting HIV infection better than another anti-HIV drug taken daily as an oral tablet. The oral tablet, called Truvada®, consists of the two anti-HIV drugs – emtricitabine and tenofovir disoproxil fumarate (TDF/FTC).
Study Schema
Sponsored By:
Division of AIDS, US National Institute of Allergy and Infectious Diseases
Pharmaceutical Support Provided By:
